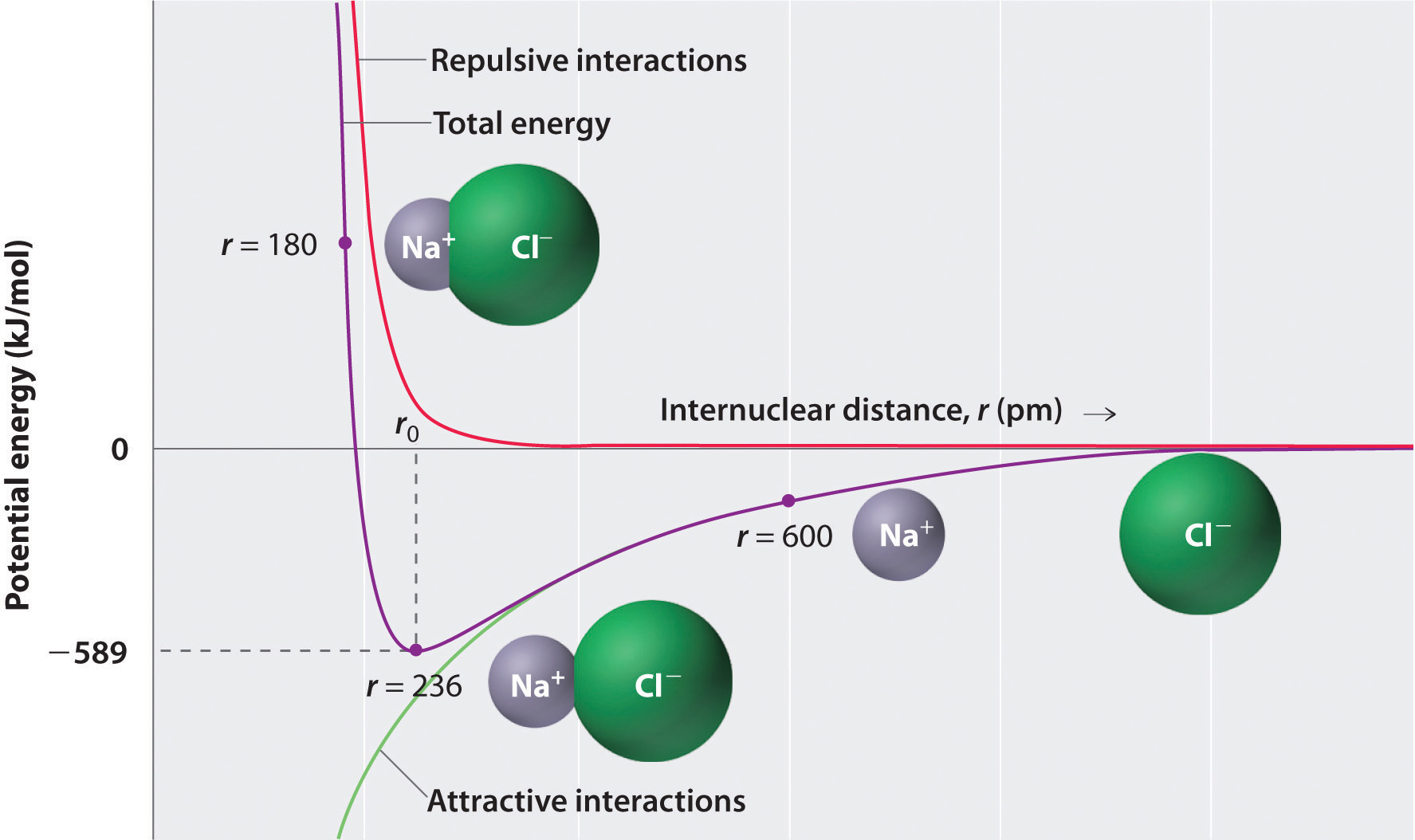
Ionic Bond Theory . Predict the charge of common metallic. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.
from chem.libretexts.org
Explain the formation of cations, anions, and ionic compounds. In ionic bonding one element is much more electronegative then another element. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a.
Chapter 4.1 Ionic Bonding Chemistry LibreTexts
Ionic Bond Theory Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.the properties of ionic compounds shed some light on the nature of ionic bonds. In other words, the electronegative atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a.
From www.slideserve.com
PPT Ionic bonding, Lewis Dot Diagrams PowerPoint Presentation, free Ionic Bond Theory Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. This exchange results in a. In other words, the electronegative atom. Ionic solids exhibit a crystalline structure and.the properties of ionic compounds shed some light on the nature of ionic bonds. Ionic Bond Theory.
From proper-cooking.info
Ionic And Covalent Bonding Cartoon Ionic Bond Theory ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. This exchange results in a. In other words, the electronegative atom. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.the properties of ionic compounds shed some light on the. Ionic Bond Theory.
From www.pinterest.com.au
Ionic Bond vs. Covalent Bond venn diagram shows the similarities and Ionic Bond Theorythe properties of ionic compounds shed some light on the nature of ionic bonds. In ionic bonding one element is much more electronegative then another element. In other words, the electronegative atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic solids exhibit a crystalline structure and. Ionic Bond Theory.
From 2012books.lardbucket.org
Ionic versus Covalent Bonding Ionic Bond Theory Ionic solids exhibit a crystalline structure and. Predict the charge of common metallic. In other words, the electronegative atom. In ionic bonding one element is much more electronegative then another element. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic Bond Theory.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID5676143 Ionic Bond Theorythe properties of ionic compounds shed some light on the nature of ionic bonds. In other words, the electronegative atom. This exchange results in a. Predict the charge of common metallic. Explain the formation of cations, anions, and ionic compounds. Ionic Bond Theory.
From postimg.cc
6 ionic bonding 01 — Postimages Ionic Bond Theory Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Explain the formation of cations, anions, and ionic compounds.the properties of ionic compounds shed some light on the nature of ionic bonds. In ionic bonding one element is much more electronegative then another element. ionic bond,. Ionic Bond Theory.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Ionic Bond Theory In ionic bonding one element is much more electronegative then another element. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic solids exhibit a crystalline structure and. In other words, the electronegative atom. This exchange results in a. Ionic Bond Theory.
From www.showme.com
Ionic bonding Science, Chemistry, Chemical Bonds ShowMe Ionic Bond Theory Explain the formation of cations, anions, and ionic compounds. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Predict the charge of common metallic. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a.the properties of ionic compounds shed. Ionic Bond Theory.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I Ionic Bond Theory Explain the formation of cations, anions, and ionic compounds. Predict the charge of common metallic. In ionic bonding one element is much more electronegative then another element. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. In other words, the electronegative atom. Ionic Bond Theory.
From www.slideserve.com
PPT Chemical Bonding Bonding Theory and Lewis Formulas PowerPoint Ionic Bond Theorythe properties of ionic compounds shed some light on the nature of ionic bonds. In other words, the electronegative atom. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic solids exhibit a crystalline structure and. In ionic bonding one element is much more electronegative then another element. Ionic Bond Theory.
From www.thoughtco.com
Examples of Ionic Bonds and Compounds Ionic Bond Theory In ionic bonding one element is much more electronegative then another element.the properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic bonding is a type of chemical bond in. Ionic Bond Theory.
From 9pdschemistry.blogspot.com
Grade 9 Chemistry 2017 Week Two Ionic Bond Theory Predict the charge of common metallic. Ionic solids exhibit a crystalline structure and.the properties of ionic compounds shed some light on the nature of ionic bonds. In other words, the electronegative atom. This exchange results in a. Ionic Bond Theory.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID6445592 Ionic Bond Theory This exchange results in a. Explain the formation of cations, anions, and ionic compounds. In ionic bonding one element is much more electronegative then another element. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. In other words, the electronegative atom. Ionic Bond Theory.
From worldnewlive.com
Where Do Ionic Bonds Always Occur? Mastery Wiki Ionic Bond Theory Ionic solids exhibit a crystalline structure and. This exchange results in a. In other words, the electronegative atom.the properties of ionic compounds shed some light on the nature of ionic bonds. Explain the formation of cations, anions, and ionic compounds. Ionic Bond Theory.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.6 Metallic Bonding翰林国际教育 Ionic Bond Theory This exchange results in a. Explain the formation of cations, anions, and ionic compounds. Ionic solids exhibit a crystalline structure and. Predict the charge of common metallic. In ionic bonding one element is much more electronegative then another element. Ionic Bond Theory.
From www.slideserve.com
PPT Chemical Bonding Bonding Theory and Lewis Formulas PowerPoint Ionic Bond Theory Explain the formation of cations, anions, and ionic compounds. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.the properties of ionic compounds shed some light on the nature of ionic bonds. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions. Ionic Bond Theory.
From www.hiclipart.com
Chemistry, Chemical Bond, Atom, Ionic Bonding, Atomic Theory, Structure Ionic Bond Theory ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a.the properties of ionic compounds shed some light on the nature of ionic bonds. This exchange results in a. Predict the charge of common metallic. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom. Ionic Bond Theory.
From proper-cooking.info
Ionic Bond Diagram Ionic Bond Theory In ionic bonding one element is much more electronegative then another element. ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a. Ionic solids exhibit a crystalline structure and. Predict the charge of common metallic. In other words, the electronegative atom. Ionic Bond Theory.